Have you ever stopped a medication because you felt worse after taking it-and then wondered if the drug was really to blame? Or maybe you were told to try the same drug again just to see if the problem comes back. That’s not just guesswork. It’s dechallenge and rechallenge-two of the most powerful tools doctors and pharmacologists use to figure out if a drug is truly causing a side effect.
What Is Dechallenge?
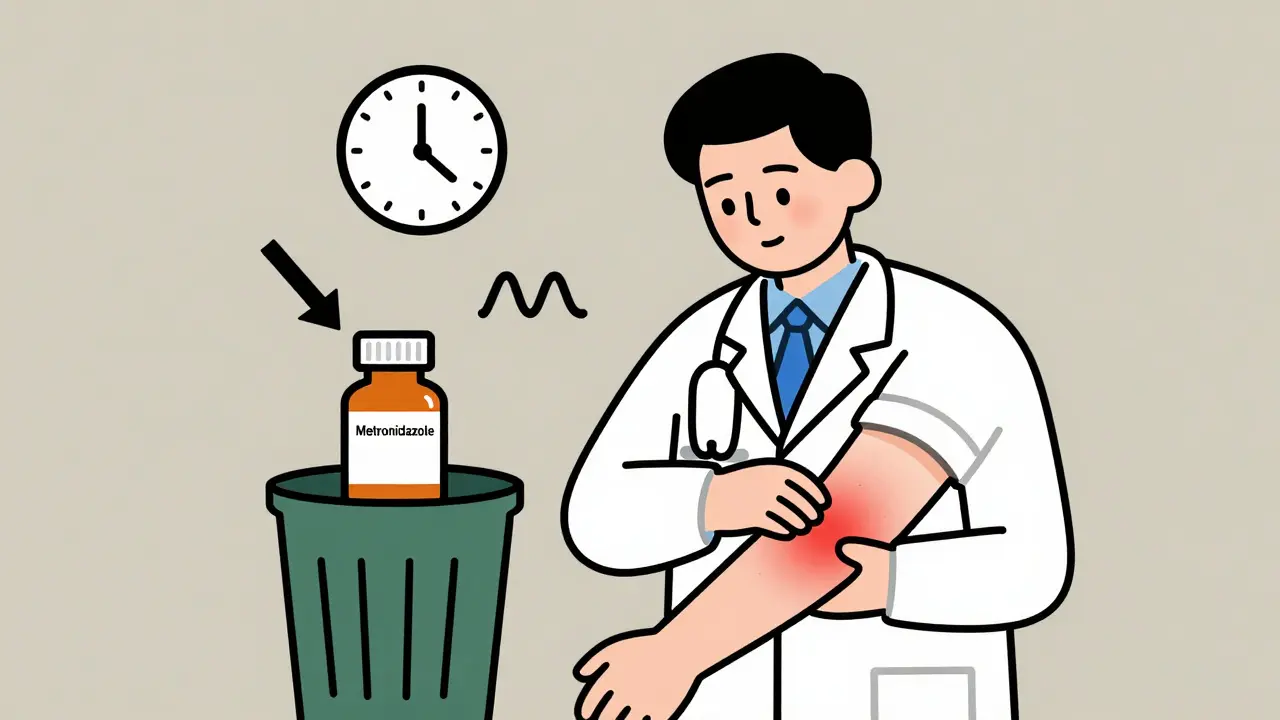
Dechallenge is simple: you stop the drug and watch what happens. If the weird rash, nausea, dizziness, or liver enzyme spike goes away, that’s a positive dechallenge. It means the drug was likely the culprit. If symptoms stick around? That’s a negative dechallenge-and the drug probably isn’t the cause, or the damage is already done.It sounds obvious, but it’s not always that clear. Take a patient on metronidazole for a bacterial infection who develops a painful, recurring skin rash. The doctor stops the drug. Two weeks later, the rash fades. The patient’s skin is still a little darkened from the inflammation, but the active reaction is gone. That’s a textbook positive dechallenge. The timing matches the drug’s half-life. The reaction didn’t vanish overnight, but it followed a predictable pattern. That’s what makes it credible.
But here’s the catch: patients often stop their meds on their own. They feel bad, so they quit. No doctor involved. No record of when they stopped. No follow-up. That’s not dechallenge-it’s self-discontinuation. And it’s useless for proving causality. Real dechallenge needs documentation: when the drug was stopped, what symptoms were present, how long they lasted, and how they improved. Without that, you’re just guessing.
What Is Rechallenge?
Now imagine this: after the rash clears, the doctor says, “Let’s try the drug again-just a small dose, under supervision.” If the rash comes back, exactly the same way, in the same spot, within a day or two? That’s a positive rechallenge. And that’s the gold standard.Rechallenge doesn’t just suggest a link-it confirms it. In fact, when a rechallenge produces the same reaction, the likelihood that the drug caused it jumps from “probable” to “definite” in global pharmacovigilance systems. The WHO-Uppsala Monitoring Centre says rechallenge alone can push causality certainty to 97% in validated cases. That’s not a small thing. It’s the difference between a hunch and a diagnosis you can trust.
But here’s the problem: rechallenge is risky. If the side effect was a life-threatening skin condition like Stevens-Johnson Syndrome, or liver failure, you don’t rechallenge. You never do. The risk is too high. Even for less severe reactions, it’s tightly controlled. Only done under medical supervision. Only with informed consent. Only after ethics boards sign off. In the U.S., fewer than 0.3% of serious adverse event investigations even consider rechallenge. It’s not common. It’s not routine. But when it’s done right, it’s the most convincing evidence you can get.
Why These Tests Matter Beyond the Individual Patient
You might think this is all about one person’s rash or stomach upset. But it’s not. Dechallenge and rechallenge are the backbone of drug safety monitoring worldwide.Every time a drug hits the market, regulators rely on reports from doctors and patients. But with hundreds of drugs, thousands of side effects, and millions of users, how do you know which reaction is real? That’s where dechallenge and rechallenge come in. They turn anecdotal reports into actionable data. If 20 different patients all show a positive dechallenge and rechallenge to the same drug after developing a rare skin reaction, that’s a signal. A red flag. And that’s how drugs get warnings added, dosing changed, or even pulled from the market.
Pharmaceutical companies are required by law to track these outcomes. The FDA and European Medicines Agency demand it. The global pharmacovigilance market-worth $12.7 billion in 2023-is built on this kind of data. In dermatology, 87% of adverse reaction assessments include dechallenge documentation. In liver injury cases, it’s 79%. In psychiatry? Only 43%. Why? Because stopping antidepressants or antipsychotics can trigger relapse. The risk of rechallenge isn’t just physical-it’s psychological. So doctors weigh it carefully.
How Doctors Use These Tools in Practice
In real clinics, dechallenge is the first step. If a patient reports a new symptom after starting a drug, the standard advice is: stop the drug and wait. Not forever. Usually 5 to 14 days, depending on how fast the drug leaves the body. A drug with a short half-life, like ibuprofen, should clear in a day or two. A drug like fluoxetine? It lingers for weeks. So the monitoring window changes.Doctors don’t just guess. They use checklists. Did the reaction start after the drug was introduced? Did it improve after stopping? Is there a known biological reason the drug could cause this? These are the four pillars of causality assessment: timing, dechallenge, rechallenge, and biological plausibility.
And now, tech is helping. Wearable sensors can track heart rate, skin temperature, and even inflammation markers in real time as a patient stops a drug. One 2023 study showed these devices captured resolution data in 78% of cases-far better than patient memory alone. That’s huge. It means less reliance on “I think it got better” and more on “Here’s the actual data.”
Still, nothing beats the clinical observation. A nurse sees the rash fade. A doctor hears the patient say, “I haven’t felt dizzy since I stopped the pill.” That’s still the most reliable evidence.
When Rechallenge Isn’t an Option
Let’s be clear: rechallenge is rare for a reason. You don’t rechallenge a drug that caused anaphylaxis. Or toxic epidermal necrolysis. Or acute liver failure. The consequences are too severe.But even for milder reactions, doctors hesitate. Why? Because patients remember. If you’ve had a bad reaction once, you’re not going to want to try it again. And if you’re the doctor, you don’t want to be the one who made it worse.
That’s why alternatives are growing. Researchers at the NIH are testing lab tests that use a patient’s own immune cells to predict if they’ll react to a drug-without ever giving them the drug. One test, called a lymphocyte toxicity assay, correctly predicted reactions in 89% of cases. That’s promising. But it’s still experimental. And it doesn’t replace the real-world proof that dechallenge and rechallenge provide.
Some places are trying AI to predict dechallenge outcomes. A machine learning model trained on thousands of past cases can estimate how long a reaction will take to clear after stopping a drug. In trials, it was 76% accurate. Useful? Yes. But it’s still a prediction. Not proof.
What You Should Know as a Patient
If you’re on a new medication and something feels off, don’t panic. But don’t ignore it either. Talk to your doctor. Don’t just stop the drug on your own. Write down when you started it. What symptoms you got. When they started. How bad they were. If you stop it, tell your doctor. Let them help you document it.If your doctor suggests rechallenge, ask why. What’s the benefit? What’s the risk? What happens if it comes back? Make sure you understand the plan. You have the right to say no.
And if you’ve had a bad reaction before, tell every new doctor. Put it in your medical records. Say: “I had a reaction to X drug. I don’t want to take it again.” That’s not being difficult. That’s being smart. And that information helps future patients too.
Final Thoughts: Why This Still Matters
We live in a world of algorithms, AI, and big data. But when it comes to figuring out if a drug made you sick, the best tool is still the simplest: stop it and see what happens. Then, if it’s safe, try it again.Dechallenge and rechallenge aren’t fancy. They don’t need expensive machines. They just need careful observation, honesty, and time. And they’re the reason we know that penicillin causes rashes, that statins can cause muscle pain, and that certain antibiotics trigger dangerous skin reactions.
These aren’t just medical terms. They’re the quiet, essential steps that keep drugs safe for everyone. You might never hear about them. But every time you take a pill without getting sick, it’s because someone, somewhere, did a dechallenge-and maybe even a rechallenge-to make sure it was safe.
Can dechallenge and rechallenge be done with any drug?
Not always. Dechallenge can be done with almost any drug if the side effect isn’t life-threatening. Rechallenge is only considered for mild or moderate reactions, and only under strict medical supervision. It’s never done for severe reactions like anaphylaxis, Stevens-Johnson Syndrome, or drug-induced liver failure because the risk is too high.
How long should I wait after stopping a drug to see if symptoms improve?
It depends on the drug. For drugs that leave your body quickly-like ibuprofen or antibiotics-you might see improvement in 1 to 3 days. For drugs with long half-lives, like fluoxetine or amiodarone, it can take 2 to 4 weeks. Doctors use the drug’s pharmacokinetics to estimate the timeline. If symptoms don’t improve within that window, the drug is less likely to be the cause.
Is rechallenge dangerous?
Yes, it can be. Rechallenge means giving you the drug again after you’ve had a bad reaction. Even if the reaction was mild before, it could be worse the second time. That’s why it’s only done in controlled settings-with emergency equipment on hand, and only after you’ve given informed consent. Most doctors avoid it unless absolutely necessary.
What if I stopped the drug on my own? Does that count as dechallenge?
No. Self-discontinuation doesn’t count because there’s no medical record of timing, symptoms, or follow-up. For dechallenge to be useful in assessing causality, it must be documented by a healthcare provider. That way, the improvement can be linked clearly to stopping the drug, not to other factors like diet, stress, or another illness.
Are there alternatives to rechallenge if it’s too risky?
Yes. Researchers are developing lab tests that use your immune cells to predict if you’ll react to a drug. One test, called a lymphocyte toxicity assay, has shown 89% accuracy in predicting reactions without re-exposure. Other tools include AI models that predict dechallenge outcomes based on past data. But none of these are yet as reliable as actual clinical observation. They’re supplements-not replacements.

Write a comment