What exactly is an authorized generic?
An authorized generic is a brand-name drug sold under a different label-same active ingredients, same factory, same quality control-but without the brand name on the bottle. It’s not a copy. It’s the exact same pill or capsule that came out of the original manufacturer’s plant, just packaged and sold as a generic. The FDA requires these to match the brand drug in every way: strength, shape, color, how fast it dissolves, and how your body absorbs it. The only difference? The price.
Why do they cost less?
They cost less because they don’t carry the marketing, advertising, and R&D costs that go into launching a brand-name drug. When a company invents a new medicine, it spends years and hundreds of millions developing it. That cost gets baked into the price. Once the patent expires, competitors can make copies. But with an authorized generic, the original company itself makes the copy. They don’t need to recoup their R&D investment anymore. So they lower the price to stay competitive.
Think of it like this: if you owned a popular coffee shop and someone opened a rival across the street, you could either fight them or open your own cheaper version under a different name. That’s what brand manufacturers do with authorized generics. They protect their market share by offering the same product at a lower price.
How much cheaper are they?
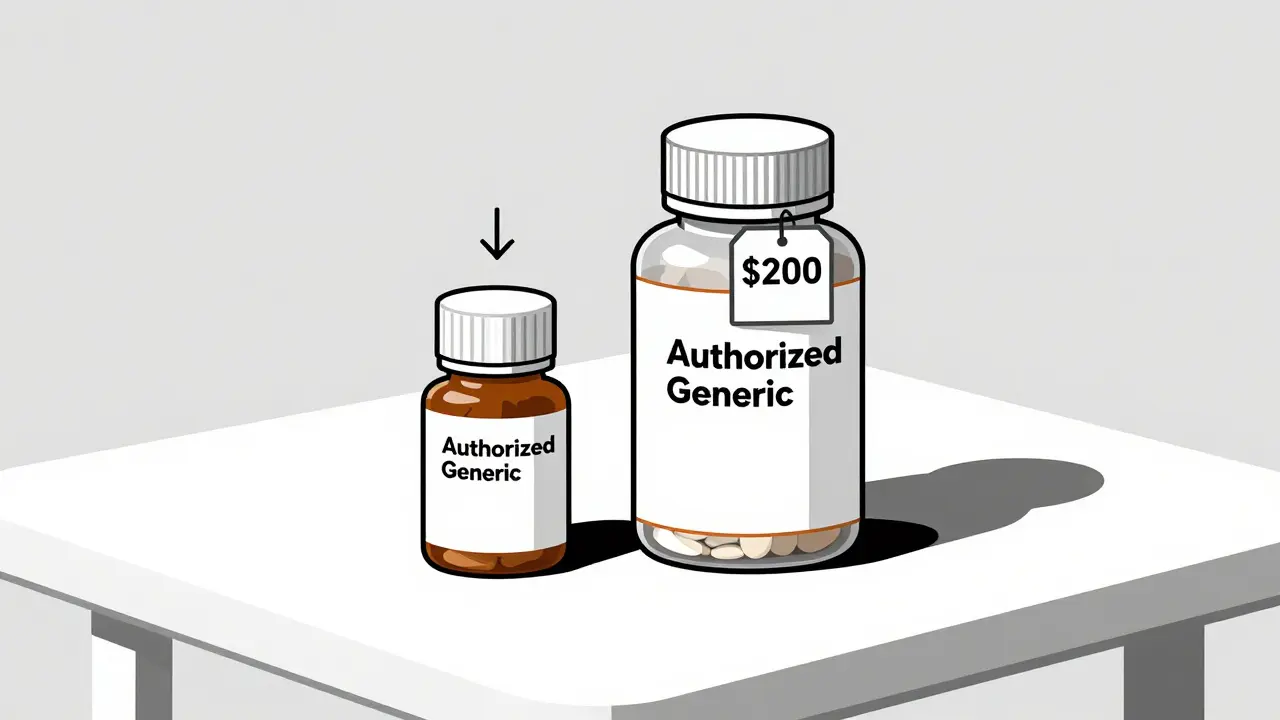
On average, authorized generics cost 4% to 8% less than the brand-name version. That might not sound like a lot, but for someone taking a medication daily, it adds up. A $200 monthly prescription drops to $184. Over a year, that’s $192 saved. For seniors on fixed incomes or people managing chronic conditions, that kind of savings matters.
But here’s the twist: sometimes the savings are even bigger. When an authorized generic hits the market at the same time as a traditional generic, it forces the first generic maker to slash prices fast. Data from Medicaid and pharmacy records show that on-invoice prices-the amount pharmacies pay-drop by 13% to 18% when an authorized generic enters the market. That means pharmacies can pass on even bigger discounts to patients.
Why don’t all generics work this way?
Regular generics are made by different companies after the brand’s patent expires. They have to go through their own FDA approval process called an ANDA. That takes time. They also need to prove their product is bioequivalent to the brand. Authorized generics skip all that. They’re made under the original brand’s FDA approval (called an NDA), so they don’t need a separate application. That’s why they can launch on day one of generic competition.
It’s like having a factory that already has the blueprint. No need to redesign. Just repackage and sell under a new name.
What’s the catch? Are they really the same?
Yes, they’re the same. The FDA requires it. The same active ingredient. The same inactive ingredients (like fillers or dyes). Same manufacturing line. Same batch testing. The only thing different is the label. Some people worry they’re getting a lower-quality version. They’re not. In fact, many pharmacists will tell you they prefer authorized generics because they know exactly where they came from.
One big myth is that authorized generics are "inferior" because they’re cheaper. That’s not true. The cost difference comes from business strategy, not quality. The same company that made the brand drug made the authorized generic. No shortcuts. No cost-cutting on materials.
Why do pharmaceutical companies use them?
It’s not just about helping patients. It’s about business. When a patent expires, the brand loses 80% of its sales within a year. Authorized generics let the original company keep a slice of that market. Instead of losing everything to a single generic competitor, they split the pie. They might even get the first-mover advantage.
Take the EpiPen. In 2016, Mylan faced huge backlash after raising the price from $100 to $600. They responded by launching an authorized generic for $300. It didn’t fix the outrage, but it gave customers a cheaper option-and kept Mylan in the game.
Another example: Gilead launched authorized generics of its hepatitis C drugs Harvoni and Epclusa before their patents even expired. Why? Because they knew competitors were coming. By offering the same drug at a lower price, they kept patients loyal and avoided losing market share to cheaper generics.
How does this affect your prescription costs?
It depends on your insurance. Pharmacy Benefit Managers (PBMs) decide which drugs go on which tier of your plan. Sometimes, they put the authorized generic on the same tier as the brand. That means you pay the same copay for both. But if they put the authorized generic on a lower tier, your out-of-pocket cost drops.
Studies show that when PBMs prioritize authorized generics over the brand, patient adherence improves by over 8%. That’s because people are more likely to fill their prescriptions when they’re cheaper. For drugs like blood pressure pills or antidepressants, that’s huge. Missing doses can lead to hospital visits-and way higher costs down the line.
But here’s the problem: PBMs don’t always make it easy to know which version you’re getting. The label might say "authorized generic," but your pharmacy receipt might just list it as "generic." Ask your pharmacist. If you’re paying the same as the brand, you might be missing out.
Are there downsides?
Some critics say authorized generics are a tactic to delay real competition. If the brand company releases its own generic, the first generic manufacturer might not get to enjoy its 180-day exclusivity period. That means fewer competitors enter the market, and prices might not drop as much in the long run.
The Federal Trade Commission has looked into this. Their data shows that while authorized generics do lower prices in the short term, they can sometimes reduce the number of traditional generic competitors. That’s why regulators are watching closely. But there’s no evidence that authorized generics cost more than other generics over time.
What should you do?
If you’re on a brand-name drug that’s gone generic, ask your doctor or pharmacist: "Is there an authorized generic?" If there is, ask your insurance if they’ll cover it at a lower tier. You might not need to switch medications-you just need to switch the label.
Check your prescription receipt. Look for the manufacturer name. If it’s the same as the brand, you’re probably getting the authorized version. Even if it doesn’t say "authorized generic," it might still be one.
And if your pharmacy says they don’t carry it, ask them to order it. Many pharmacies can get it in within a day. Authorized generics are widely available for common medications like statins, blood pressure pills, and antidepressants.
Bottom line
Authorized generics aren’t a loophole. They’re a legal, FDA-approved way to get the exact same medicine for less. They’re not cheaper because they’re worse. They’re cheaper because the company doesn’t need to charge for marketing, patents, or decades of development anymore. For patients, they’re a rare win-win: same drug, same results, lower price.
Don’t assume generics are all the same. Ask. Compare. Save.

Write a comment