When you pick up a generic drug at the pharmacy, you expect it to work just like the brand-name version. You’re not just saving money-you’re trusting that the pill in your hand is as safe and effective as the one your doctor originally prescribed. But how does the FDA make sure that’s true? It’s not magic. It’s a detailed, science-backed system of checks that starts long before the drug hits the shelf and continues long after.
How Generic Drugs Get Approved
Generic drugs don’t need to repeat the same clinical trials as brand-name drugs. That’s because the FDA already confirmed the safety and effectiveness of the original drug. Instead, manufacturers must prove their version is bioequivalent. That means the generic drug delivers the same amount of active ingredient into your bloodstream at the same rate as the brand. The FDA requires this range to be between 90% and 110%-no more, no less.
This is done through an Abbreviated New Drug Application, or ANDA. The ANDA isn’t short on detail. It includes everything from the chemical structure of the drug to how it’s made, stored, and tested. The FDA reviews every piece of data, including lab results, manufacturing processes, and stability studies. If something doesn’t meet the standard, the application gets a “complete response letter” and must be resubmitted with more data. This back-and-forth can take months, sometimes over a year.
The Role of cGMP in Drug Safety
Even if a drug works perfectly in a lab, it can still be dangerous if it’s made in a dirty or poorly controlled facility. That’s why the FDA enforces Current Good Manufacturing Practices, or cGMP. These aren’t suggestions-they’re legal requirements.
cGMP covers three critical areas:
- Raw materials: Every ingredient, even the smallest one, must be tested for purity and identity. Contaminated chemicals or incorrect powders can ruin an entire batch.
- Production controls: Every step in the manufacturing process has written procedures. Temperature, mixing time, pressure-all are monitored and recorded. If a machine goes out of spec, the batch is rejected.
- Quality testing: Finished pills are tested for strength, dissolution, and contamination. Labs use validated methods that meet international standards. No shortcuts.
These rules apply equally to factories in Ohio and those in India or China. The FDA doesn’t trust geography-it trusts data.
Inspections: The Eyes on the Ground
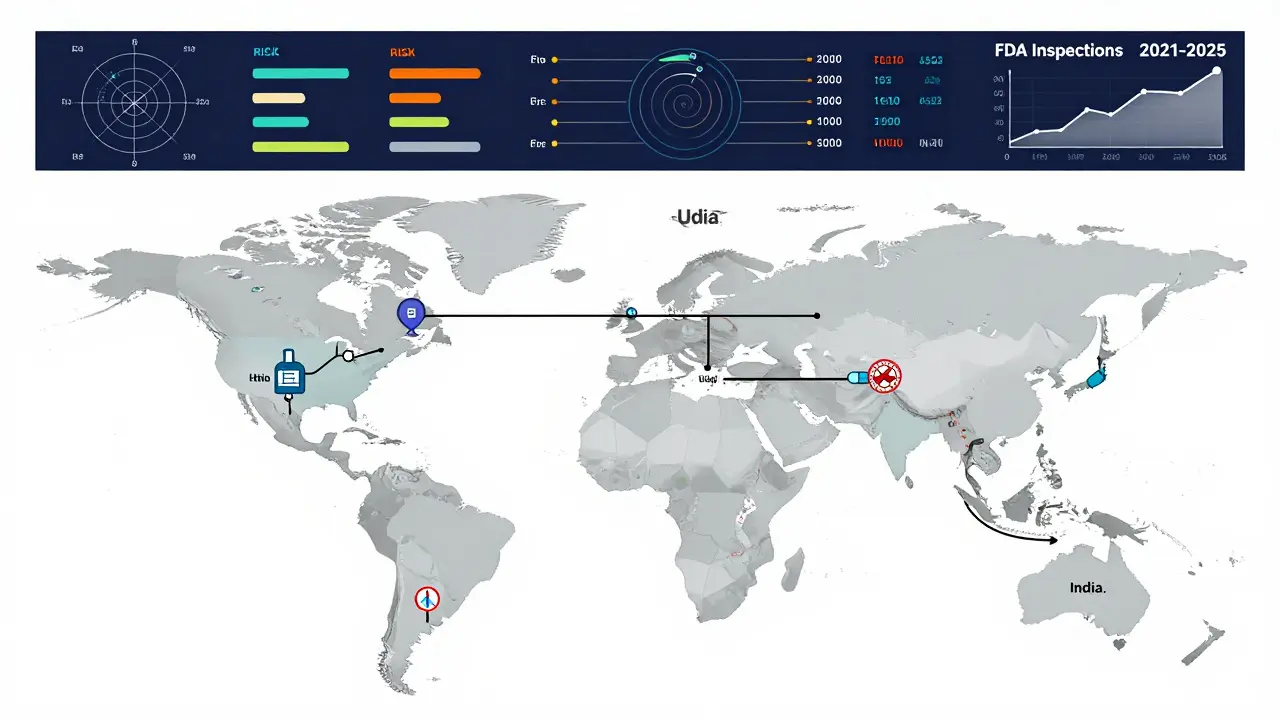
Documents can be forged. Data can be manipulated. That’s why the FDA sends inspectors-real people with clipboards and sampling kits-to manufacturing sites. In 2021, the agency conducted 1,082 inspections worldwide. By 2025, that number is expected to hit 1,500.
Inspections aren’t scheduled like a dentist appointment. They’re risk-based. A facility with past violations, a history of data issues, or one that produces complex drugs like inhalers or injectables gets priority. The FDA also looks at where the ingredients come from. Many active pharmaceutical ingredients (APIs) are made overseas, so inspectors follow the supply chain.
In 2019, 15% of foreign facilities had serious quality issues. Domestic facilities? Only 8%. That gap hasn’t disappeared, but it’s narrowing. Thanks to the Generic Drug User Fee Amendments (GDUFA), the FDA now has the funding to hire more inspectors and use better tools-like real-time data monitoring and risk algorithms-to target the highest-risk sites.

Post-Market Surveillance: Watching After Approval
Approval doesn’t mean the job is done. The FDA keeps watching. Every year, over 1.3 million adverse event reports come in through MedWatch. These reports come from patients, pharmacists, and doctors. If a pattern emerges-say, multiple reports of nausea or dizziness linked to a specific generic drug-the FDA investigates.
The Office of Generic Drugs works with the Office of Pharmaceutical Quality and the Division of Clinical Safety and Surveillance to analyze these signals. They don’t wait for a crisis. They use proactive data mining to spot anomalies in drug usage, packaging errors, or unexpected side effects. If a problem is confirmed, the FDA can require a label update, issue a public warning, or even ask the company to recall the product.
One example: in 2020, a batch of generic metformin was found to contain a cancer-causing impurity called NDMA. The FDA didn’t wait for thousands of reports. It acted quickly, tested multiple manufacturers, and issued recalls within weeks.
Why This System Works
Generic drugs make up 90% of prescriptions in the U.S. but only cost 23% of total drug spending. That’s over $313 billion saved every year. But that’s only possible because the FDA’s oversight system is strict.
It’s not perfect. There have been lapses. In 2015, the Office of Inspector General found the FDA hadn’t completed all requested preapproval inspections. But the system has evolved. GDUFA I (2012), GDUFA II (2018), and GDUFA III (2022) have poured over $1 billion into modernizing inspections, speeding up reviews, and improving global oversight.
The FDA now reviews 95% of standard ANDAs in under 10 months-down from 30 months in the early 2010s. That’s not because they’re cutting corners. It’s because they’ve invested in better tools, more staff, and smarter processes.
What’s Next for Generic Drug Safety
The FDA is now focusing on complex generics-drugs that are hard to copy, like inhalers, patches, and injectables. These require different testing methods and more advanced manufacturing. The agency has released over 2,800 scientific guidance documents since 2018, helping manufacturers get it right the first time.
Also, the Drug Supply Chain Security Act (DSCSA) now requires electronic tracking of every prescription drug from factory to pharmacy. That means if something goes wrong, the FDA can trace it back to the exact batch, the exact machine, and the exact shift.
And the goal hasn’t changed: every generic drug on the shelf must meet the same standard as the brand name. No exceptions. No compromises.
Why You Can Trust Generic Drugs
You might hear rumors that generics are less effective or unsafe. But the data says otherwise. The FDA has reviewed over 1,100 generic drugs in a single year. Each one was held to the same standard as the original. And when safety issues arise, they’re caught-not because someone got lucky, but because the system is designed to catch them.
Generic drugs are not cheap because they’re low quality. They’re cheap because they don’t need to repeat expensive clinical trials. The science, the inspections, the surveillance-all of it is there. The FDA doesn’t cut corners. It just cuts the waste.


Write a comment